Inherited retinal disorders—or IRDs—are disorders that affect the retina, the part of the eye that sees light, leading to severe vision loss or blindness. There are many IRDs and each one is caused by a gene variant that affects how the retina functions. Learn why gene therapy has strong potential to slow or stop the progression of these diseases, and helping save vision.
LUXTURNA is an FDA-approved gene therapy for the treatment of adults with an inherited retinal disorder affecting the RPE65 gene.
About IRDs
Cause of disease- Inherited retinal disorders (IRDs) are caused by a gene variant, also known as a gene mutation, which is a change in one or more of our genes. This change makes cells in the retina not work as needed and causes vision loss. There are many genes known to cause IRDs with new ones still being discovered. These disorders affect individuals of all ages, with different types progressing at different rates. Many are degenerative, which means the disease worsens over time. Common types of IRDs include: Leber Congenital Amaurosis (LCA), Retinitis Pigmentosa, Choroideremia, Stargardt’s Disease, and Achromatopsia.
Diagnosis- Depending on the kind of disorder or gene variant, an IRD can show up at different times of a person’s life. It is good practice to see an eye doctor on a regular basis, who can help detect early signs of a serious retinal disorder. IRDs can cause irreversible damage, so early diagnosis provides the best chance of keeping vision. People can consider genetic testing to identify a gene variant causing their disorder, or to understand if they carry a gene variant that could be passed along to their child.
Gene Therapy Approach
Gene therapy aims to target the underlying cause of disease, the gene variant for an IRD. The treatment aims to be given one time, compared to other treatments for retinal disease that need to be given more frequently.
There are a variety of ways gene therapy can be given or delivered into the eye. Most gene therapy approaches use a viral vector to deliver the genetic material, such as a working gene, into the cells. Viruses can be used as vectors because they have evolved to be very good at getting into cells. There are two kinds of injections into the eye that gene therapy may be given. Intravitreal injections are performed by directly injecting the therapy into the vitreous, a jelly-like fluid near the retina. Subretinal injections are administered underneath the retina into the subretinal space, allowing the therapy to be closer to the target tissue of the eye. Suprachoroidal injections can also be used to better reach the back part of the eye. No matter the route, gene therapies for IRDs are typically given at Ocular Gene Therapy Treatment Centers or other specially trained sites.
Why Gene Therapy for IRDs?
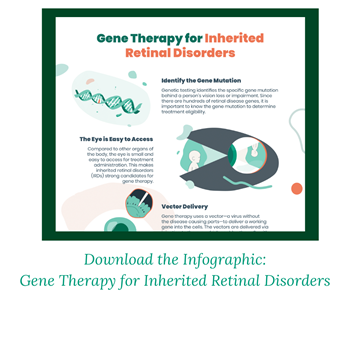 Gene therapy is a strong option for IRDs, since most of them are caused by genetic variants or changes. The treatment being given directly into the eye allows more efficient delivery to the target tissues and lowers potential exposure to other body systems. The eye is also considered an “immune privileged” site which means it is protected from our immune system. Usually when a foreign substance—like a virus—enters our bodies, our immune system would attack it. However, certain areas of the body are immune privileged, which means our normal immune response isn’t as active. This is typically in areas of our bodies that are very important and may become damaged if swelling or inflammation occurs, such as the eyes. When gene therapy is given to the affected tissues in the eyes, there is lower likelihood it will be rejected from an immune system attack. This makes it more likely to provide therapeutic benefits to slow or stop the progression of the disorder.
Gene therapy is a strong option for IRDs, since most of them are caused by genetic variants or changes. The treatment being given directly into the eye allows more efficient delivery to the target tissues and lowers potential exposure to other body systems. The eye is also considered an “immune privileged” site which means it is protected from our immune system. Usually when a foreign substance—like a virus—enters our bodies, our immune system would attack it. However, certain areas of the body are immune privileged, which means our normal immune response isn’t as active. This is typically in areas of our bodies that are very important and may become damaged if swelling or inflammation occurs, such as the eyes. When gene therapy is given to the affected tissues in the eyes, there is lower likelihood it will be rejected from an immune system attack. This makes it more likely to provide therapeutic benefits to slow or stop the progression of the disorder.
Current Treatments
FDA-approved Luxturna is the first approved gene therapy for an inherited retinal disorder in the U.S. It is also approved for use in Europe. Luxturna delivers a working copy of the RPE65 gene into the eye. Speak with your ophthalmologist or a healthcare provider to determine if you may be appropriate for treatment with Luxturna by confirming mutations (or variants) in both copies of the RPE65 gene.
Treatment Pipeline
There are gene therapy approaches for various IRDs that are currently in preclinical studies and clinical trials. Clinical trials are a required part of the research process that aims to understand the way a drug or treatment will interact with the human body and whether it is safe and effective. Preclinical studies are an even earlier stage of research to confirm the safety and effectiveness of a treatment in animal or cell-based models before proceeding with a human clinical trial. Clinical trials may differ on various aspects of their design. Speak with a trusted provider or member of the clinical trial research team if you are considering participating in a clinical trial.
To stay up to date on active and recruiting clinical trials that may become available in the U.S. or globally, visit ClinicalTrials.gov or the Gene Therapy Trial Browser.
Participating in a Clinical Trial
It is important to be well informed when deciding to participate in a clinical trial or receive an approved treatment. Below are some key points to consider. Go to the Clinical trials process or considering a clinical trial pages for more information and resources to help guide you.
- Eligibility-Eligibility for a trial is based on strict inclusion and exclusion criteria, such as age, medical history, and disease severity. It is important to keep in mind that IRDs are caused by different gene variants, and therefore each clinical trial has different eligibility criteria. Genetic testing is necessary to identify a person’s specific variant, since more than 260 retinal disease genes have been found. If you think you or a loved one may be eligible for a clinical trial, it is best to first speak with a trusted provider or a member of the research team to determine if it is right for you.
- Risks-As with any medical intervention, there are risks that need to be carefully considered. Before participating in a clinical trial, a member of the research team should review any potential risks and benefits with the patient or caregiver. Therapies being studied in clinical trials are not a guaranteed cure and cannot guarantee beneficial results. There is always a chance that the investigational treatment may not work. In the event a person is not satisfied with the outcome, the person may not receive another dose of the gene therapy. In addition, participating in a clinical trial may prevent future participation in other trials or from receiving other types of treatments. Gene therapy can be an alteration for the lifetime, so people should be aware that there could be long term effects (both good or bad) that are not known at this time.
- Benefits-Participating in a trial may offer many potential benefits compared to not receiving any form of intervention for a fatal disease. Gene therapy aims to be a one-time treatment with lasting positive effects that slow or stop disease progression for a lifetime. However, there is no guarantee. If gene therapy is received earlier in the course of disease, it has the potential to stop damage before it occurs.
- Long-term follow up-If receiving a current approved therapy such as Luxturna, the treatment will be administered one time and require frequent follow up visits with those who administered the treatment within a general post operation period. Those participants should plan on follow-up visits with a doctor who specializes in the retina at least once a year.
For those who participate in a clinal trial, it is the individual’s responsibility to comply with the long term follow up. The Food and Drug Administration (FDA) guidelines require the clinical trial research team to monitor safety and potential long-term effects of a gene therapy. Follow-up may require in-person appointments that vary in frequency and location, or completion of mailed packets with response forms. The need for long-term data collection for a gene therapy trial can last up to 15 years—another reason to consider all outcomes and responsibilities that come with committing to a clinical trial. There are a limited number of participants in trials so a lack of attendance at follow-up appointments leads to not enough study data. This could negatively affect FDA approval of a new therapy and thereby limit access to the therapy by patients who did not participate in the clinical trial.
Access
At this time, we do not know if or when more gene therapies will be approved by the FDA and commercially available for people living with different types of IRDs. The overall process may take several more years, until it is deemed safe and effective by the FDA or regulatory agencies in other countries.
Stay Informed
There are many patient advocacy organizations to follow or get involved with. They work hard to fund research and advocate for patient and family needs. Many patients suffering from IRDs are unawae that treatment options are in development, and many may even be misdiagnose. They are also a great way to connect with others affected by the disease if you are looking for support and advice.