The use of messenger RNA (mRNA) in medical interventions has been researched for the last 20 years, including in vaccines hoping to fight diseases such as cancer, rabies, Ebola and the Zika virus. That means the research was ready to be applied to this new disease, COVID-19. With the recent approval of an mRNA vaccine, Comirnaty, along with emergency use of another to fight COVID-19, we can build protection against the virus in our communities if enough people are vaccinated. Lean more about how the vaccine works and find answers to many common questions below.
What are mRNA vaccines and how do they work to defend against COVID-19?
UPDATE: On August 23, 2021 the FDA approved the vaccine that has been known as the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 disease in individuals 16 years of age and older. The vaccine will now be marketed as Comirnaty. This vaccine will continue to be available under emergency use authorization for individuals 12 through 15 years of age and for the administration of a third dose in certain people who are immunocompromised.
What is COVID-19?
 COVID-19 is an infectious disease caused by a strain of coronavirus discovered in 2019, called SARS CoV-2. The disease is typically spread through respiratory droplets in the air from an infected person. SARS CoV-2 causes a range from no symptoms (asymptomatic) to severe symptoms, including fever, cough, fatigue, and breathing difficulty. Coronaviruses are a family of viruses that cause a number of different diseases, including COVID-19. The COVID-19 outbreak is defined as a pandemic, because it has spread across multiple countries and has affected a large number of people.
COVID-19 is an infectious disease caused by a strain of coronavirus discovered in 2019, called SARS CoV-2. The disease is typically spread through respiratory droplets in the air from an infected person. SARS CoV-2 causes a range from no symptoms (asymptomatic) to severe symptoms, including fever, cough, fatigue, and breathing difficulty. Coronaviruses are a family of viruses that cause a number of different diseases, including COVID-19. The COVID-19 outbreak is defined as a pandemic, because it has spread across multiple countries and has affected a large number of people.
What is mRNA?
Messenger RNA, or mRNA, are molecules within the body that contain genetic instructions for cells to make proteins that are required for the body to function properly.
How does an mRNA vaccine work?
.aspx?width=350&height=350) MRNA vaccines deliver synthetic mRNA molecules into cells, instructing them to make antigens. An antigen is a foreign invader that the immune system recognizes as not being part of itself, such as the protein surface of a virus. In the case of the COVID-19 mRNA vaccines, cells are instructed to only make the SARS Cov-2 spike protein, which is just enough to activate the immune system. But the cells are not given enough instructions to make a full virus, so the vaccine cannot cause COVID-19. Unlike conventional vaccines, mRNA vaccines do not use a weakened or killed virus. These antigens then trigger the immune system to produce specific protective antibodies that neutralize the virus–in this case, the antibodies needed to fight COVID-19. The mRNA in Comirnaty is only present in the body for a short time and does not alter the recipient’s genetic material. If a person is exposed to COVID-19, the immune system will detect the familiar antigens and produce antibodies to attack them. A vaccinated person’s immune system can better defend against the infection altogether or greatly reduce the severity of the infection.
MRNA vaccines deliver synthetic mRNA molecules into cells, instructing them to make antigens. An antigen is a foreign invader that the immune system recognizes as not being part of itself, such as the protein surface of a virus. In the case of the COVID-19 mRNA vaccines, cells are instructed to only make the SARS Cov-2 spike protein, which is just enough to activate the immune system. But the cells are not given enough instructions to make a full virus, so the vaccine cannot cause COVID-19. Unlike conventional vaccines, mRNA vaccines do not use a weakened or killed virus. These antigens then trigger the immune system to produce specific protective antibodies that neutralize the virus–in this case, the antibodies needed to fight COVID-19. The mRNA in Comirnaty is only present in the body for a short time and does not alter the recipient’s genetic material. If a person is exposed to COVID-19, the immune system will detect the familiar antigens and produce antibodies to attack them. A vaccinated person’s immune system can better defend against the infection altogether or greatly reduce the severity of the infection.
How many doses of the mRNA vaccine are required?
The two mRNA vaccines for COVID-19 available in the U.S. require two doses, three to four weeks apart, to be effective. With these vaccines, if the body only encounters the antigen once, it’s unclear how long the protection would last. Encountering the same antigen again in a short time boosts the abundance of protective antibodies on standby, so people are safeguarded for as long as possible.
What are clinical trials?
Clinical trials are a required step in the research process that studies the way an intervention interacts with the body. Clinical trials typically take many years and are divided into different phases that answer specific questions about the treatment, primarily whether it is safe and effective. However, the FDA has created expedited pathways and programs to accelerate the process while still maintaining high standards. This is typically done for drugs and therapies for serious diseases with no other treatment options, or for public health emergencies— fitting for interventions that fight COVID-19.
Who is eligible to receive the vaccine?
Everyone 16 years and older is now eligible for COVID-19 vaccines. The vaccine Comirnaty is also authorized for emergency use for individuals 12 to 15 years of age and for the administration of a third does in certain people who are immunocompromised.
Do I still need to get vaccinated if I already had COVID-19?
Yes. The CDC recommends getting vaccinated even if you have already had COVID-19, because you can get infected more than once. You may have short-term antibodies for protection after recovering from COVID-19, but we don’t know how long this protection lasts. Also, there were no concerns reported from the clinical trials if a participant had COVID-19 and was also vaccinated.
Are there certain people who should not receive the vaccine?
Very few, but individuals with known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the COVID-19 vaccine should not receive the vaccine. Speak with your doctor to make the decision if:
- You are immunocompromised, meaning you have a known impaired immune system*
- You are pregnant or breastfeeding**
- You have a history of severe allergic reaction such as anaphylaxis to any vaccine or injectable therapy
*See the CDC recommendations on COVID-19 vaccines for people who are moderately to severely immunocompromised.
**See the CDC Recommendations on COVID-19 vaccines while pregnant or breastfeeding.
If I have a rare disease, will the mRNA vaccine affect my eligibility to receive gene therapy in the future?
If you are immunocompromised, speak with your provider to make the decision about getting vaccinated. However, there is no use of a virus or viral vector with the mRNA vaccines, so receiving an mRNA vaccine will not prevent you from obtaining a gene therapy in the future for your rare disease.
If I am currently a participant in a gene therapy clinical trial, can I get the vaccine?
Check with your provider of the gene therapy trial before receiving the vaccine.
How effective are the mRNA vaccines?
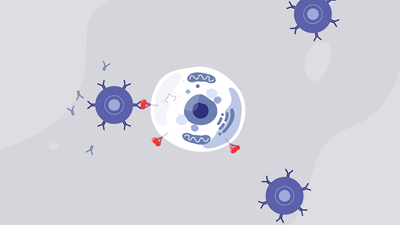
mRNA instructs the cells to create the spike protein to trigger antibody production.
Comirnaty Vaccine: To support the approval decision of Comirnaty, the FDA reviewed updated data from the clinical trial that supported the EUA and included a longer duration of follow up in a larger clinical trial population. The FDA analyzed effectiveness data from approximately 20,000 vaccine and 20,000 placebo recipients who were 16 and older and did not have evidence of infection within a week of receiving the second dose. The Agency evaluated the safety of Comirnaty in approximately 22,000 people who were 16 and older and received the vaccine, and 22,000 people who received a placebo. Results from the clinical trial showed the vaccine was 91% effective in preventing COVID-19. More than half of the clinical trial participants were followed for safety outcomes for at least four months after the second dose. Overall, approximately 12,000 recipients have been followed for at least six months. The vaccine is effective in preventing COVID-19 and potentially serious outcomes including hospitalization and death.
Data supporting EUA: Based on clinical trials, researchers and health experts have found the results to be positive. The mRNA vaccines were shown to reduce the risk of adults getting COVID-19 by over 90 percent.
Moderna’s study involved more than 30,000 participants in the U.S. and was 94.5 percent effective at reducing the risk of getting COVID-19, with only five cases found in the vaccinated group versus 90 in the non-vaccinated group. Racially and ethnically diverse backgrounds were achieved, making up 37 percent of U.S. participants. Almost 25 percent of participants were over the age of 65, and just over 15 percent of people had high-risk medical conditions like diabetes and heart disease.
Pfizer and BioNTech’s vaccine involved over 43,000 participants globally and was 95 percent effective at reducing the risk of getting COVID-19, with only eight cases appearing in the vaccinated group versus 162 in the non-vaccinated group. Racially and ethnically diverse backgrounds were achieved, making up 30 percent of U.S. participants and 42 percent of participants in other countries. The trial also had participants in the high-risk age group of 56-85 years old, which made up 45 percent of U.S. participants and 41 percent globally.
Are there any side effects of the COVID-19 vaccine?
Protective antibodies fight off COVID-19.
Like any medical intervention, vaccines can cause side effects, which are known from studies to be caused by the vaccine. This often means that your immune system is doing its job and any mild symptoms typically last only a few days. Side effects are a type of adverse event. An adverse event is any health problem ranging from minor to serious, that happens after vaccination, whether it is caused by the vaccine or not.
Comirnaty Vaccine: The most commonly reported side effects by the clinical trial participants who received Comirnaty were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever. Serious adverse events considered by FDA to be plausibly related to the vaccine or vaccination procedure were one case of shoulder injury at the vaccination site and one case of swollen lymph node in the armpit opposite the vaccination arm. Data on Comirnaty demonstrates increased risks of myocarditis and pericarditis following administration, particularly within the seven days following the second dose. Available data from short-term follow-up suggest that most individuals have had resolution of symptoms. However, some individuals required intensive care support. Information is not yet available about potential long-term health outcomes.
Data supporting EUA: During testing in the early clinical trials to obtain the emergency use authorization, mRNA vaccines had not caused any serious adverse events. There have since been isolated incidents of rapid onset of allergic reaction, and of facial swelling in participants who had previously received botox; all cases were treatable, and the participants have recovered. Sites that are administering vaccines are now required to have appropriate medical treatments immediately available for severe allergic reactions. Moderna announced that less than 10 percent of participants who received the vaccine reported short-lived feelings of fatigue, headache, achiness, and muscle pain. The Pfizer and BioNTech vaccine also resulted in no serious safety concerns observed, with less than 4 percent of participants reporting fatigue or headache. Call your doctor if you have any symptoms that concern you after you are vaccinated.
How long does the vaccine provide protection?
At this time, data is not available to determine how long the vaccine will provide protection. However, having protection for any length of time from the vaccine is beneficial over no protection. Getting vaccinated also protects people around you, particularly people at increased risk for severe illness from COVID-19.
Will the vaccine stop me from spreading the virus to others?
At this time, there is no evidence of whether the vaccine prevents transmission of SARS-CoV-2 from person to person. Clinical trial data show that these adenovirus-based vaccines do a very good job at preventing symptomatic COVID-19. But data is still being collected to understand how well the vaccine prevents asymptomatic infection (infection without symptoms). Research is ongoing to determine whether a vaccinated person might still be able to transmit COVID-19 to someone else who is not vaccinated. This is why it is important for a large proportion of the population to be vaccinated.
How does a vaccine help build herd immunity?
 Vaccinations and herd immunity are not two separate approaches to fighting a pandemic. Herd immunity occurs when a large portion of a community (the herd) becomes immune to a disease, making the spread of disease from person to person unlikely. Vaccines are used to help populations reach herd immunity faster and without spreading illness. Advancements in vaccines are some of the greatest public health achievements over the last century. The only way we can build enough protection against the virus in our communities is if the majority of people receive the vaccine.
Vaccinations and herd immunity are not two separate approaches to fighting a pandemic. Herd immunity occurs when a large portion of a community (the herd) becomes immune to a disease, making the spread of disease from person to person unlikely. Vaccines are used to help populations reach herd immunity faster and without spreading illness. Advancements in vaccines are some of the greatest public health achievements over the last century. The only way we can build enough protection against the virus in our communities is if the majority of people receive the vaccine.
How was the mRNA vaccine produced and tested so quickly?
Lipid nanoparticles encase the mRNA to protect it before it enters the cell.
The mRNA vaccines for COVID-19 have been developed at a record-setting speed, but that doesn’t mean that they aren’t tested to the same safety and efficacy standards as other medical interventions. Before it is made widely available, any vaccine needs to be first studied in clinical trials. Then it is reviewed by an agency that oversees the safety and effectiveness of medical products. In the United States, this is done by the Food and Drug Administration (FDA). Starting a clinical trial for any medical intervention requires a research team, adequate funding and resources, and enough initial patients and data to properly plan the study. It’s a daunting task and is often why research and development take so long. Because this disease is a global health emergency, funding has been quickly directed toward reducing many of these barriers. The goal is to treat this dangerous disease as soon as possible, and as COVID-19 infection rates increased through 2020, researchers were able to gather a lot of data and eager volunteers for clinical trials.
What is Emergency Use Authorization?
During a declared public health emergency, such as the COVID-19 pandemic, the FDA can issue an Emergency Use Authorization (EUA) to provide access to medical products that may be used when there are no adequate, approved, and available alternatives. Under an EUA, the FDA makes a product available to the public based on the best available evidence, without waiting for all the evidence that would be needed for FDA approval. When evaluating an EUA application, the FDA carefully balances the potential risks and benefits of the products. The vaccines that have been granted EUA status in the United States are the mRNA vaccine developed by Moderna, the mRNA vaccine developed by Pfizer/BioNTech, and the adenovirus-based vaccine developed by J & J.
UPDATE: On August 23, 2021 the FDA approved the vaccine known as Pfizer-BioNTech COVID-19 vaccine and will now be marketed as Comirnaty for the prevention of COVID-19 disease in individuals aged 16 years of age and older.
How do the available vaccines compare?
In the U.S. there is one approved mRNA vaccine, as well as an mRNA vaccine and an adenovirus-based vaccine that are authorized for emergency use for COVID-19 from the FDA. The adenovirus-based vaccine does not need to be kept as cold as the mRNA vaccines, which makes it easier to distribute while the mRNA vaccines are easier to make compared to the adenovirus-based vaccine, which needs a viral vector to deliver genes to the cells. All the vaccines have provided protection against COVID-related hospitalizations and deaths.
Where can I find additional information?
The Centers for Disease Control and Prevention (CDC),the U.S. Food and Drug Administration (FDA), and the World Health Organization (WHO) offer credible resources about COVID-19 and mRNA vaccines for COVID-19.
Was this information helpful? If so, please share! All ASGCT Patient Education resources are free to use by sharing on social media, embedding the video, or simply linking to this page! Please credit The American Society of Gene and Cell Therapy.