The average length of time from symptom onset of a rare disease to an accurate diagnosis is nearly five years. Along the diagnostic journey, a person may see around seven different medical specialists before a receiving a diagnosis. Genetic testing, in a variety of forms, is one way to identify rare genetic diseases, which can determine if a person has a disease caused by a change (mutation) in a gene.
Finding the Right Specialist
 An important step in the journey towards obtaining an accurate diagnosis is finding the correct specialist. It's possible that one person may encounter many medical specialists prior to diagnosis and during their disease course. A primary care provider can often provide referrals to relevant specialists. Depending on your symptoms, this could be a hematologist, oncologist, movement disorders specialist, neurologist, rheumatologist, and for some, a medical geneticist or genetic counselor. It’s also recommended to contact your insurance company to find a professional in your area who is part of your plan.
An important step in the journey towards obtaining an accurate diagnosis is finding the correct specialist. It's possible that one person may encounter many medical specialists prior to diagnosis and during their disease course. A primary care provider can often provide referrals to relevant specialists. Depending on your symptoms, this could be a hematologist, oncologist, movement disorders specialist, neurologist, rheumatologist, and for some, a medical geneticist or genetic counselor. It’s also recommended to contact your insurance company to find a professional in your area who is part of your plan.
- The National Society of Genetic Counselors has also created a helpful directory to assist physicians, patients and other counselors in accessing services.
- Genetics Home Reference also has put together a comprehensive guide to finding a genetics professional that is right for your specific needs.
- For those who are already on this diagnostic journey but have found it challenging to get answers, consider The Undiagnosed Diseases Network. This is a research study funded by the National Institutes of Health Common Fund that patients and families can apply for which brings together clinical and research experts to solve challenging medical mysteries using advanced technologies.
Understanding Genetic Inheritance
Before we talk about the different kinds of tests, let’s first discuss how genetic diseases are passed down. Typically, everyone has two copies of each gene, one from their biological mother and one from their biological father. A carrier is someone who only has one mutated copy of the gene, typically showing no symptoms for the disease.
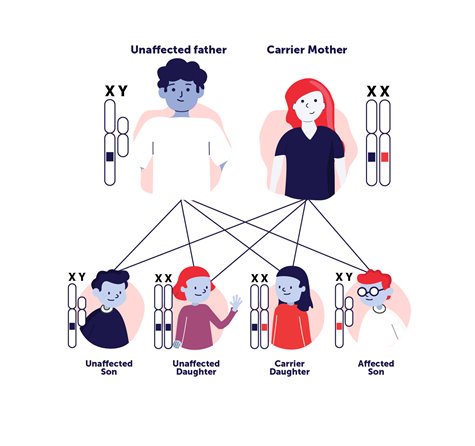 For recessive disorders it takes two mutated copies of the gene for a person to have a genetic disorder. However, carriers may pass along the disorder to their child—if both parents are recessive carriers of a mutated gene. Autosomal dominant is a pattern of inheriting a genetic mutation that is located on one of the non-sex, numbered chromosomes (autosomal) and a single copy of the disease-associated mutation from one parent is enough to cause disease (dominant). X-linked inheritance (see image at left) means the genetic mutation is located on the X-chromosome which can be recessive or dominant inheritance. There are also de novo (new) mutations that can sometimes explain genetic disorders in which an affected child has mutations, but the parents do not and there is no family history of the disorder.
For recessive disorders it takes two mutated copies of the gene for a person to have a genetic disorder. However, carriers may pass along the disorder to their child—if both parents are recessive carriers of a mutated gene. Autosomal dominant is a pattern of inheriting a genetic mutation that is located on one of the non-sex, numbered chromosomes (autosomal) and a single copy of the disease-associated mutation from one parent is enough to cause disease (dominant). X-linked inheritance (see image at left) means the genetic mutation is located on the X-chromosome which can be recessive or dominant inheritance. There are also de novo (new) mutations that can sometimes explain genetic disorders in which an affected child has mutations, but the parents do not and there is no family history of the disorder.
Genetic Testing
Genetic testing is a medical test that can sometimes identify the genetic aspects of disease. This information can be used to develop a plan to manage and sometimes treat the condition and to provide information about whether a condition could be passed along to one’s children.
Treatment options may include approved gene therapies or eligibility for clinical trials. Genetic testing may be done to look for mutations in specific genes, and it’s important to remember that a negative test result means that a disease-causing mutation was not found in any of the genes examined. It does not rule out all possible genetic disorders. However, genetic testing can rule out specific diagnoses and better inform a patient’s approach to medical care and additional testing moving forward.
Screening for Genetic Conditions at Birth
A common form of genetic testing is newborn screening. Newborn babies are routinely screened for certain genetic disorders and conditions during the first days of life, and iswhich is required in every state before the baby leaves the hospital. The test is part of standard care, and parents do not need to request newborn screening.
Newborn screening is administered by a prick to the baby’s heel, where a few drops of blood are collected. It’s worth noting that there can be false positive screenings. Therefore, if a child's test is positive, testing will be done to confirm the diagnosis, and if a diagnosis is made, then treatment options are considered right away.
 Each state decides which conditions to include in its newborn screening program and not all genetic conditions are included in newborn screening panels. To learn about your state’s policies and which conditions are tested for, visit Baby’s First Test. People can also reference the Recommended Uniform Screening Panel (RUSP) which is a list of disorders that an advisory committee to the U.S. Department of Health and Human Services recommends states screen for. For all of these disorders, patient advocates can play a role in advancing this important public health service in two main ways. First, you can nominate a disorder for inclusion on the RUSP. Patient advocacy groups interested in completing this process should reach out to the Committee for the RUSP directly to increase the chane of a successful application. Second, you can support efforts to add disorders to your state's standard screening panel. Some states add disorders through the legislative process, while others do so through administrative changes. There's not one approach that applies to every state, so start by contacting your state-level elected representative or seeking out ogranizations that have been involved in previous efforts.
Each state decides which conditions to include in its newborn screening program and not all genetic conditions are included in newborn screening panels. To learn about your state’s policies and which conditions are tested for, visit Baby’s First Test. People can also reference the Recommended Uniform Screening Panel (RUSP) which is a list of disorders that an advisory committee to the U.S. Department of Health and Human Services recommends states screen for. For all of these disorders, patient advocates can play a role in advancing this important public health service in two main ways. First, you can nominate a disorder for inclusion on the RUSP. Patient advocacy groups interested in completing this process should reach out to the Committee for the RUSP directly to increase the chane of a successful application. Second, you can support efforts to add disorders to your state's standard screening panel. Some states add disorders through the legislative process, while others do so through administrative changes. There's not one approach that applies to every state, so start by contacting your state-level elected representative or seeking out ogranizations that have been involved in previous efforts.
For many diseases screened at birth, the earlier the testing, the better, as a treatment can be pursued before irreversible damage occurs. For example, spinal muscle atrophy (SMA) is screened for in some states, so if a child is diagnosed then FDA-approved treatments for SMA can be administered as soon as possible.
Carrier Screenings
A carrier screen is a genetic test that determines if an individual is carrying a copy of the mutated gene known to cause disease; for example, sickle cell disease or spinal muscle atrophy (SMA). Being a carrier of a genetic disease is not uncommon. Most of the time, carriers don’t know that they are carrying a gene for a disorder and aren’t currently showing symptoms.
 Carrier screenings are usually done with a blood test. A person will work with their doctor or genetic counselor to determine the most appropriate tests. A carrier screen is a voluntary decision, but all women who are thinking of becoming or are already pregnant can be screened for certain diseases. Since many disorders take two carriers (both parents) to produce a child with that disorder, if one is determined to be a carrier, then it is likely the other will be tested as well. There are targeted screenings based on family history or ethnicity, and expanded screening options that may test for as many as 100 different disorders. This is because some disorders have a higher incidence based on ethnicity, like sickle cell disease, which is more common in people of African or Mediterranean descent.
Carrier screenings are usually done with a blood test. A person will work with their doctor or genetic counselor to determine the most appropriate tests. A carrier screen is a voluntary decision, but all women who are thinking of becoming or are already pregnant can be screened for certain diseases. Since many disorders take two carriers (both parents) to produce a child with that disorder, if one is determined to be a carrier, then it is likely the other will be tested as well. There are targeted screenings based on family history or ethnicity, and expanded screening options that may test for as many as 100 different disorders. This is because some disorders have a higher incidence based on ethnicity, like sickle cell disease, which is more common in people of African or Mediterranean descent.
Once the results return, a doctor or qualified medical professional will review them with the patient and family, or recommend a meeting with a genetic counselor. These are professionals that can interpret carrier screening results on a deeper level and interpret family history, analyze current risks and predict future ones.
Genome Sequencing
New advances in genome sequencing can help doctors diagnose genetic diseases. In the past, sequencing technology allowed researchers to sequence short pieces of DNA. However, the process was expensive, time consuming, and only provided a small snapshot of a person’s g enetic makeup. Next-generation sequencing technologies have given clinicians the ability to obtain a sequence of an entire human genome for a patient in a number of days or weeks.
enetic makeup. Next-generation sequencing technologies have given clinicians the ability to obtain a sequence of an entire human genome for a patient in a number of days or weeks.
Genome sequencing determines the order of the four chemical building blocks, called bases, that make up the DNA molecule. Looking for mutations or changes in that order which are known to cause a genetic disease can yield a diagnosis. Sometimes genome sequencing of parents, as well as the child, can help determine a child’s diagnosis. This more thorough approach to genetic testing can be helpful in cracking a more complicated diagnostic journey. If a diagnosis is found, doctors can try to connect the family with clinical experts and researchers studying that disease.