There are a variety of ways to find a clinical trial, such as through an internet search, online database, or through conversations with a healthcare professional or patient organization. Unfortunately, there are some trials that have not received the necessary regulatory approval, so it is important to be attentive and critical when evaluating clinical trial listings. For example, the clinical trials listed on ClinicalTrials.gov are entered by the trial sponsors, and are not vetted by the National Institute of Health (NIH). Illegitimate trials are few and far between, but can have serious consequences, making it important to understand how to find a promising trial and being aware of red flags for an illegitimate trial.
There are a variety of ways to find a clinical trial, such as through an internet search, online database, or through conversations with a healthcare professional or patient organization. Unfortunately, there are some trials that have not received the necessary regulatory approval, so it is important to be attentive and critical when evaluating clinical trial listings. For example, the clinical trials listed on ClinicalTrials.gov are entered by the trial sponsors, and are not vetted by the National Institute of Health (NIH). Illegitimate trials are few and far between, but can have serious consequences, making it important to understand how to find a promising trial and being aware of red flags for an illegitimate trial.
The ASGCT Clinical Trials Finder is an excellent resource and is updated daily to be a user-friendly, searchable database to find all active and recruiting trials for gene and cell therapies internationally. However, if it is your first time navigating the database, you may want to know what specific information is provided and why it is relevant. This guide, provided by the Courageous Patient Network, can also assist in understanding common terms used related to clinical trials.
Trial Summary
Below is a view of an active clinical trial within the ASGCT Clinical Trials Finder. The Lead Sponsor is an individual, organization or institution that initiates, manages and finances a clinical trial. Oftentimes a trial has Additional Sponsors, who help with funding, data collection or other responsibilities. The Phase refers to the step that the trial is on. Typically, a trial has three research phases, and sometimes these phases are combined, but each phase progresses the research to enroll a specific number of patients with the hope of answering a different research question. When a person takes part in a clinical trial, they will only be in that one phase of the study. Sometimes, phases of a clinical trial are combined, which results in making the evaluation process more efficient and making the treatment available faster.
The green Open icon indicates that the trial is currently active. Age indicates the age range of eligible participants, and the Enrollment Goal outlines the desired number of participants for the specific phase. Interventions indicate that the trial divides participants into different groups, receiving different interventions (treatments) related to the disease. Modalities are different methods of medical treatment, like surgery, cell therapy, gene therapy, or chemotherapy. Clinical trial listings will also include the Start date of the trial and the date of the Last Update to the clinical trial’s listing. Each listing has a Purpose Summary that explains the big picture goal of a study and its specifics, and an ID Number assigned to it by the institute, agency, or organization sponsoring the trial that is linked to the original listing on clinicaltrials.gov.

Eligibility Criteria
There are a variety of Eligibility Criteria for clinical trials, including factors such as age range, status of disease progression and past treatments. Pictured below, inclusion criteria are listed as minimum criteria that need to be met to qualify, while exclusion criteria indicate factors that disqualify someone from a study. Watch ASGCT Secretary Terence Flotte, M.D., discuss more on eligibility criteria.

Arms, Interventions, Outcome Measures
The Arms and Interventions section refers to how certain groups of participants will receive potentially different therapies. Certain phases of clinical trials may have two or more treatments, tested on different groups to see different outcomes. For example, one group may receive a standard treatment and the other may receive an investigational treatment to compare outcomes. This section of the listing outlines the research approach, timing, and the specifics of what the treatment consists of.
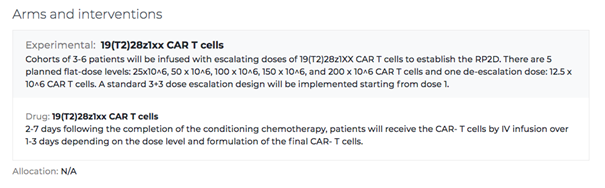
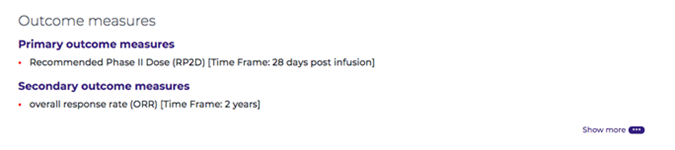
After a patient has received the therapy, Outcome Measures are measurements that are used to assess and quantify the effects (both positive or negative) of a treatment. For example, an outcome measure for a gene therapy treatment might be to analyze whether an enzyme or protein that was lacking has since increased in the bloodstream.
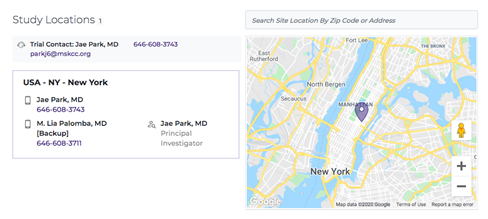
Study Location
A clinical trial for gene and cell therapy usually takes place in a hospital or clinic affiliated with an academic institution that has specialized training at limited locations since the treatment is not yet on the market. The Study Location is outlined within the trial listing, along with relevant contact information for the primary investigator or trial coordinator, which is the appropriate contact if you are interested in participating or learning more.